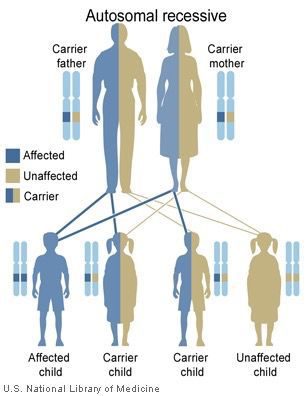
 Figure 1. Autosomal recessive inheritance pattern (National Library of Medicine (US), 2014)
Figure 1. Autosomal recessive inheritance pattern (National Library of Medicine (US), 2014)
Maple Syrup Urine Disease: A Study of Carrier Frequency in Mennonite and Non-Mennonite Populations
Many diseases are caused by genetic disorders in humans. Genetic disorders can be caused by many different genetic mutations, such as base substitutions, aneuploidy, or frameshifts. Further, mutations can be carried and passed on to offspring. Maple syrup urine disease is an autosomal recessive genetic disease which causes maple syrup urine odor, hypoglycemia, and a failure to catabolize leucine, isoleucine, and valine. The purpose of my study is to look at the carrier frequency of MSUD in Mennonite and non-Mennonite populations. DNA was collected and isolated from 96 participants. From this, PCR products and restriction enzyme digests were obtained. Gels were then run to test the participants for the presence of a mutation diagnostic of MSUD. Of the 48 Mennonite participants, 2 were found to be carriers, yielding a carrier frequency of 4.17%, and of the 48 non-Mennonite participants, zero were found to be carriers, yielding a carrier frequency of 0%. These results suggest the sampled Mennonite population is more genetically isolated than the non-Mennonite population. Due to these results, it is important for Mennonites to understand the possible consequences and risks of genetic isolation. Genetic testing and outbreeding are possible ways to combat these genetic consequences.
Many diseases can be linked to genetic origins. Base substitutions are a common cause of genetic diseases. This study looks at the chromosome region 6q14.1 (6 is the chromosome number, q is the lower arm of the chromosome, and 14.1 is the specific region). When an asparagine to tyrosine substitution occurs in this specific region, it can cause branched-chain ketoaciduria, which is more commonly referred to as maple syrup urine disease (MSUD). Even if an individual does not show the phenotype for MSUD, they could carry a mutated allele. Alleles are versions of genes. Each human has 2 alleles for every gene. MSUD occurs more often in Old Order Mennonite and Mennonite populations because of the smaller gene pools and higher rates of inbreeding. Inbreeding causes a higher rate of homozygous zygotes, and many genetic disorders are a result of homozygous recessive mutations. The Clinic for Special Children in Lancaster County, Pennsylvania is dedicated to genetic counseling and research for Old Order Mennonite and Amish families with genetic disorders. The clinic currently serves patients with 127 known disorders, one of which is MSUD (Clinic, 2014). Maple syrup urine disease is an inherited autosomal recessive disorder (see Figure 1) that occurs when the body cannot process certain amino acids (Genetics, 2014). Although maple syrup urine disease is rare in infants worldwide (1 in 185,000 infants), it occurs at the much higher rate of 1 in 358 infants in Old Order Mennonite populations (Puffenberger, 2003). Furthermore, it has a carrier frequency of 7.96% in Mennonite populations (Puffenberger, 2003). Being a carrier means having 1 mutated allele, but this mutation is not expressed because recessive disorders are only expressed when both alleles have the same mutation; that is when the alleles are homozygous. If 2 carriers mate, their offspring would express the mutation 1 out of every 4 times and would carry the mutation 1 out of every 2 times. Researching carrier frequency and genetic mutations can help scientists better understand what causes these mutations and help develop better techniques to test individuals who exhibit a mutation or carry a mutation.
Genetic disorders account for many different diseases and mutations of the human body. One type of mutation is a single-base or point mutation in which a single base is replaced by another base. A sub-type of point mutation is a transversion in which a purine is replaced by a pyrimidine or vice-versa. In a missense mutation, the replaced nucleotide alters the codon to produce a different amino acid in the protein, which is what occurs in maple syrup urine disease. Most changes in DNA are inherited from parents, and only 15-20% of genetic diseases occur during a fetus’s development (Copstead and Banaski, 2010).
Polymerase chain reaction (PCR) is a technique that has advanced molecular biology possibly more than any other technique (Carr and Moore, 2012). PCR is used to amplify specific segments of DNA through cycles of denaturation, annealing, and extension as shown in Figure 2. PCR utilizes forward and reverse primers to locate certain regions of DNA to copy (Weaver, 2012). Primers are especially useful when wanting to focus on a small particular region of a chromosome.
| Restriction Site Amplicon | Forward Primer | Reverse Primer |
|---|---|---|
| Sca1 | 5’-GTGGGCTGAGCAGGTCTCACT-3’ | 5’-CCACCTGCAGACCTACGGGGAGTAT-3’ |
| Fok1 | 5’-GGAAGCCCAAACCCAACCCCAACCT-3’ | 5’-ATCGAAGTGATCCAGTGGGA-3’ |
Restriction enzymes are enzymes that cut DNA at specific points known as restriction sites. Restriction enzymes only recognize very specific base sequences to cleave in the DNA, which makes them useful when trying to cut DNA at specific locations on a chromosome. One restriction enzyme familiar to many people who have worked with DNA is EcoRI (Loenen et al., 2014). Primers used in PCR can amplify a region known to contain a certain restriction site. A specific restriction enzyme can then be used to cut the DNA at that precise restriction site. From this, you can determine if a person has a mutation by whether or not a certain restriction enzyme cuts the amplified region of DNA.
Maple syrup urine disease is an autosomal recessive genetic disorder that results from an inability to catabolize the branched-chain amino acids leucine, isoleucine, and valine. The disease affects 1 in 185,000 births worldwide (Kniffin, 2012). The carrier frequency for the general population is 0.465% (Eldemann et al., 2001). MSUD is caused by a mutation in the branched chain keto acid dehydrogenase E1 encoded by the alpha polypeptide gene. MSUD can be caused by a homozygous or compound heterozygous mutation. There are five different clinical variants of the genotype including: classic severe, intermittent, intermediate, thiamine-responsive form, and dihydrolipoyl dehydrogenase (E3)-deficient (Kniffin, 2012). Symptoms of the disease can affect the abdomen, central nervous system, metabolism, and laboratory tests. The most prominent feature of the disease is the maple syrup odor to urine of the infants diagnosed with MSUD. Other features can include pancreatitis, vomiting, seizures, cerebral edema, hallucinations, ketosis, and hypoglycemia. If an infant with MSUD is left untreated, the symptoms worsen with seizures, coma, and death within the first months of life. Some states have screening for MSUD within the first 24 hours after birth in which blood samples are taken to check for elevated leucine levels (Genetic Science, 2014).
There are several treatments available for patients with MSUD. A dietary restriction of the branched-chain amino acids is used as soon as a patient is diagnosed to prevent brain damage. Patients must avoid food with a high content of protein, such as meat and eggs. Hemodialysis is occasionally used to remove the branched-chain amino acids from the body. Treatment of episodes of acute metabolic decompensation is also necessary for patients. When these episodes occur, glucose infusions are initiated as rapidly as possible in addition to insulin to promote anabolism (Bodamer, 2014).
Each clinical variation of MSUD varies in severity and clinical manifestations. Additionally, several different mutations lead to different variations. For example, in an Ashkenazi Jewish population, a guanine to cytosine mutation causes the manifestation of MSUD (Edelmann et al., 2001). In the Mennonite population, however, the mutation is caused by an asparagine to tyrosine substitution (Mitsubuchi et al., 1992). Numerous studies have looked at the relationship between Old Order Mennonites and high rates of maple syrup urine disease. The study conducted by Mitsubuchi, et al. in 1992 looked at Mennonite patients with clinical manifestations of MSUD. They looked at 8 different Mennonite pedigrees with patients known to have MSUD. By also testing parents, siblings, and parents’ siblings, they could find the carrier rate as well as the rate of homozygous and heterozygous mutations. Further, they developed techniques for primer-specific restriction maps to locate the asparagine to tyrosine substitution at amino acid 394 (Mitsubuchi, et al., 1992).
Another study focused on the population genetics of the Old Order Mennonites of Lancaster County, Pennsylvania and MSUD. Over a 10-year time period, 19/6,810 Old Order Mennonite births from the Groffdale and Weaverland conferences displayed MSUD. The Groffdale and Weaverland conferences had incidence rates of 1/271 and 1/686, respectively (Puffenberger, 2003). Additionally, they found genetic drift to have a major effect on the genetic disease rate. Genetic drift is a change in allele frequency due to random fluctuations that tends to have greater effects in small populations. Over time, genetic drift results in the loss of an allele or its fixation at 100% within the population. The population size and initial allele frequencies determine how quickly this occurs (Brooker, 2012). Due to the inbreeding of the Old Order Mennonite population, the frequency of homozygous genotypes increased and the rate of heterozygous genotypes decreased, which led to a higher incidence of the disease within the population.
Mennonite ancestry has always been of interest to me, and by combining genetics and Mennonites, I am able to look at how these two seemingly different topics can intersect. Old Order Mennonites have been used to study many different genetic disorders because of small gene pools, and after researching different diseases, I decided to focus on maple syrup urine disease. Other diseases considered for study were Fragile X syndrome, Hirschsprung disease, and Salla disease. MSUD had a higher frequency in Old Order Mennonites than many of the other diseases, so MSUD was chosen for this particular study. My study looks at the carrier frequency of MSUD in an ethnically Mennonite and non-Mennonite population. By looking at the carrier frequency in a Mennonite population—not an Old Order Mennonite population—we can see whether Mennonite populations around Kansas and South Dakota have small enough gene pools to also carry higher than normal carrier frequency rates of MSUD as compared to a population not connected to Mennonites. Mennonite gene pools are typically not as static as Old Order Mennonite populations. This study examines the carrier frequency of MSUD in Mennonite and non-Mennonite populations in order to compare the results to known carrier frequencies associated with the Old Order Mennonite populations and general populations. Mennonite populations are expected to have a higher carrier frequency than non-Mennonite populations but a lower carrier frequency than Old Order Mennonite populations.
Buccal swabs were collected from 48 Mennonite and 48 non-Mennonite participants. An institutional review board (IRB) protocol was used, and all participants signed a release form. Mennonite participants had multiple generations of Mennonite heritage whereas non-Mennonite participants had no known Mennonite ancestors. None of the participants were known to have or carry MSUD.
Gentra’s Purgene Cell Kit (Cat. No. 158767) was used to perform DNA isolation from buccal swabs obtained from each participant. Before the samples were used in the PCR, DNA isolation was ensured by running the samples in an electrophoresis of 0.8% agarose gel made with TAE buffer. After DNA isolation was confirmed, samples were stored at -20°C until they were used for PCR.
After DNA isolation, a Quiagen PCR Kit (Cat No. 201223) containing dNTPs, buffer, MgCl, and Taq was used. Previous researchers created forward and reverse primers to amplify the specific regions of DNA in which the Fok1 and Sca1 recognition sites are located, and these primers were used in the PCR (Mitsubuchi et al., 1992). The following thermal profile was used for the Fok1 restriction site amplicon: an initial cycle of 95°C for 3 min, followed by 40 cycles of 94°C for 35 sec, 62°C for 15 sec, and 72°C for 26 sec, and a final cycle of 72°C for 2 min. The following thermal profile was used for the Sca1 restriction site amplicon: an initial cycle of 95°C for 3 min, followed by 40 cycles of 94°C for 35 sec, 64°C for 15 sec, and 72°C for 26 sec, and a final cycle of 72°C for 2 min. Each participant’s sample had two separate PCR reactions because of the different primers used for the different amplicons containing the different restriction sites for each of the 2 restriction enzymes. Table 1 shows each forward and reverse primer used in the PCR. In order to ensure the PCR worked, each sample was run on a 1.0% agarose gel. Each gel had a 200 bp ladder on the end wells with samples in the middle wells.
The final procedure to determine whether a participant was a carrier for MSUD was the restriction enzyme digest. Each DNA sample had two restriction enzyme reactions—one with the Fok1 restriction enzyme and one with the Sca1 restriction enzyme. After incubation, the samples were loaded into polyacrylamide gels (see Figure 3). A 20 bp ladder was used on the end wells of each gel for scoring. All Fok1 reactions were run together, and all Sca1 reactions were run together for easy comparison.
If a participant carried a MSUD mutation, the sample with the Fok1 restriction enzyme was cut and formed bands at 31, 106, and 137 bp, and the sample with the Sca1 restriction enzyme was not cut. If a participant did not carry the mutation, the sample with the Sca1 restriction enzyme was cut and formed bands at 24, 44, and 68 bp, and the sample with the Fok1 restriction enzyme was not cut. These gels were used to record the results of the study. Figure 4 is a graphical representation of the band profile produced by each restriction enzyme.
Restriction enzyme digests were first run on 3.0% agarose gels. The resolution using 3.0% gels was too low, and some gels showed bands whereas other gels did not. In order to troubleshoot this problem, a restriction enzyme digest trial was conducted to find the optimum time for digestion in the 37°C water bath. The results from this trial were run on a polyacrylamide gel, which showed bands at a much higher resolution. As a result, each following restriction enzyme digest was run on polyacrylamide gel and scored by using a 20 bp ladder. Every sample had restriction enzyme specific digest patterns. The DNA samples digested with Fok1 all had the same band sizes, and all DNA samples digested with Sca1 had the same band sizes. Two Mennonite samples resulted in bands that are consistent with MSUD carriers. The non-Mennonite sample yielded zero carriers. The scored results are shown in appendix A. The carrier frequency of the Mennonite population was 2/48 = 4.17%. The carrier frequency of the non-Mennonite population was 0/48 = 0%.
A carrier frequency of 4.17% for MSUD was found in the Mennonite population and a carrier frequency of 0% was found in the non-Mennonite population. The carrier frequency of MUSD is 4.17% in the Mennonite population whereas the known carrier frequency is 7.96% in Old Order Mennonite populations (Puffenberger, 2003). The Mennonite population had a higher carrier frequency than the non-Mennonite population but a lower carrier frequency than the Old Order Mennonite population.
The Mennonite population is likely to be less genetically isolated than Old Order Mennonite populations but more potentially genetically isolated than the non-Mennonites based on the differences in carrier frequency. Consequently, it is important to understand the possible consequences and risks of genetic isolation. Inbreeding increases the number of homozygous offspring, which increases the incidence of recessive disorders. Genetic testing could prove to be beneficial for Mennonite populations. Through genetic testing, Mennonites could become aware of mutations they possess as well as the chance of passing on those mutations to their offspring. The two participants in this study who are carriers would have no way of knowing they are carriers without having participated in the study. In addition to genetic testing, outbreeding within the Mennonite population could help lower the carrier frequencies and the amount of genetic mutations within the population. In the past, Mennonites have tended to marry and produce offspring within their own Mennonite church or community. However, more people have begun to marry people outside the Mennonite church. Outbreeding can greatly decrease the incidence of homozygous recessive diseases in the population. Outbreeding also increases genetic diversity and expands the gene pool.
The participants of this study did not have any known genetic mutations. Participants were selected based on a known family history of Mennonite or non-Mennonite origins. No other criterion was used when selecting participants. It would have been ideal to use Mennonite participants all from one small town in order to obtain a potentially smaller gene pool and more consistency throughout the Mennonite sample. Additionally, an ideal study would obtain samples from every Mennonite, which was impossible because of resources and time. However, a larger sample size of between 150-200 Mennonites would provide a more accurate carrier frequency.
Some DNA isolation gels run on a 0.8% agarose gel resulted in DNA bands at different places on the gel. These results are believed to be caused by contamination or DNA degradation. Fortunately, this did not affect the PCR or restriction enzyme digest reactions. Polyacrylamide gels showed the clearest results for scoring bands of the restriction enzyme digests than any agarose gel used. The resolution of polyacrylamide gels was much higher than that of 3.0% agarose gels. Some unexpected bands occurred in the restriction enzyme digests. These bands are most likely the result of the higher resolution polyacrylamide gel or mispriming. These bands did not alter the results but were scored and recorded for accuracy.
My findings suggest the MSUD carrier frequency of Mennonite populations (4.17%) is lower than the carrier frequency of Old Order Mennonite populations (7.96%) but higher than the general population (0.465%).
Thank you to everyone who helped and supported me throughout this study! I would like to thank the following: the Bethel College biology department for providing a laboratory and equipment for this study, Dr. Francisca Méndez-Harclerode for support, insight, and knowledge throughout the study, and Dr. Brian Ammann at the Center for Disease Control for helping gain access to several scholarly articles used as references for this project. Finally, I would like to thank the URICA committee and the Mennonite Contributions Contest Endowment for helping fund this research.
Bodamer, O.A. (2014). Maple Syrup Urine Disease Treatment & Management. Medscape. Retrieved from http://emedicine.medscape.com/article/946234-treatment.
Brooker, R.J. (2012). Genetics: Analysis and Principles 4th ed. New York, NY: McGraw Hill.
Carr, A.C. and Moore, S.D. (2012). Robust Quantification of Polymerase Chain Reactions Using Global Fitting. Plos One. 7(5). Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3365123/.
Clinic for Special Children. (2014). Research. Retrieved from https://clinicforspecialchildren.org/research/.
Copstead, L.C. and Banaski, J.L. (2010). Pathophysiology 4th ed. St. Louis, MO: Saunders Elsevier.
Eldemann, L., Wasserstein, M.P., Kornreich, R., Sansaricq, C., Snyderman, S.E., and Diaz, G.A. (2001). Maple Syrup Urine Disease: Identification and Carrier-Frequency Determination of a Novel Founded Mutation in the Ashkenazi Jewish Population. Am J Hum Genet. 69(4):863-868. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1226071/.
Genetics Home Reference. (2014). Maple syrup urine disease. Retrieved from http://ghr.nlm.nih.gov/condition/maple-syrup-urine-disease.
Genetic Science Learning Center. (2014). Maple Syrup Urine Disease (MSUD). Learn Genetics. Retrieved from http://learn.genetics.utah.edu/content/disorders/singlegene/msud/.
Kniffin, C.L. (2012). Maple Syrup Urine Disease. Online Mendelian Inheritance in Man. #248600. Retrieved from http://omim.org/clinicalSynopsis/248600.
Loenen W.A., Dryden D.T., Raleigh E.A., Wilson G.G., and Murray N.E. (2014). Highlights of the DNA cutters: a short history of the restriction enzymes. Nucleic Acids Res. 42(1):3–19. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3874209/.
Mitsubuchi, H., Matsuda, I., Nobukuni, Y., Heidenreich, R., Indo, Y., Endo, F., Mallee, J., and Segal, S. (1992). Gene Analysis of Mennonite Maple Syrup Urine Disease Kindred Using Primer-Specified Restriction Map Modification. J Inherit Metab Dis. 15(2):181-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1356170.
National Library of Medicine Genetics Home Reference. (2014). Bethesda, MD: The Library. Autosomal recessive. Retrieved from http://ghr.nlm.nih.gov/handbook/inheritance/inheritancepatterns.
Puffenberger, E. G. (2003). Genetic heritage of the Old Order Mennonites of Southeastern Pennsylvania. Am J Med Genet C Semin Med Genet. 121C:18–31. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12888983.
University of Miami College of Arts and Sciences. (2014). Miami, FL: Department of Biology. Polymerase chain reaction. Retrieved from http://www.as.miami.edu/research/.
Weaver, R. F. (2012). Molecular Biology 5th ed. New York, NY: McGraw Hill.